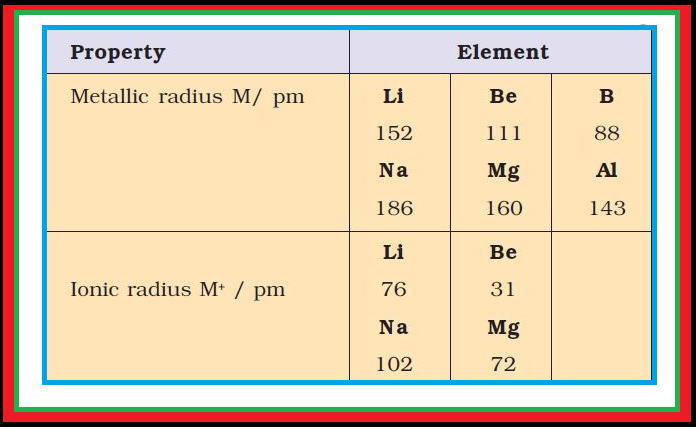
Periodicity of Valence or Oxidation States :
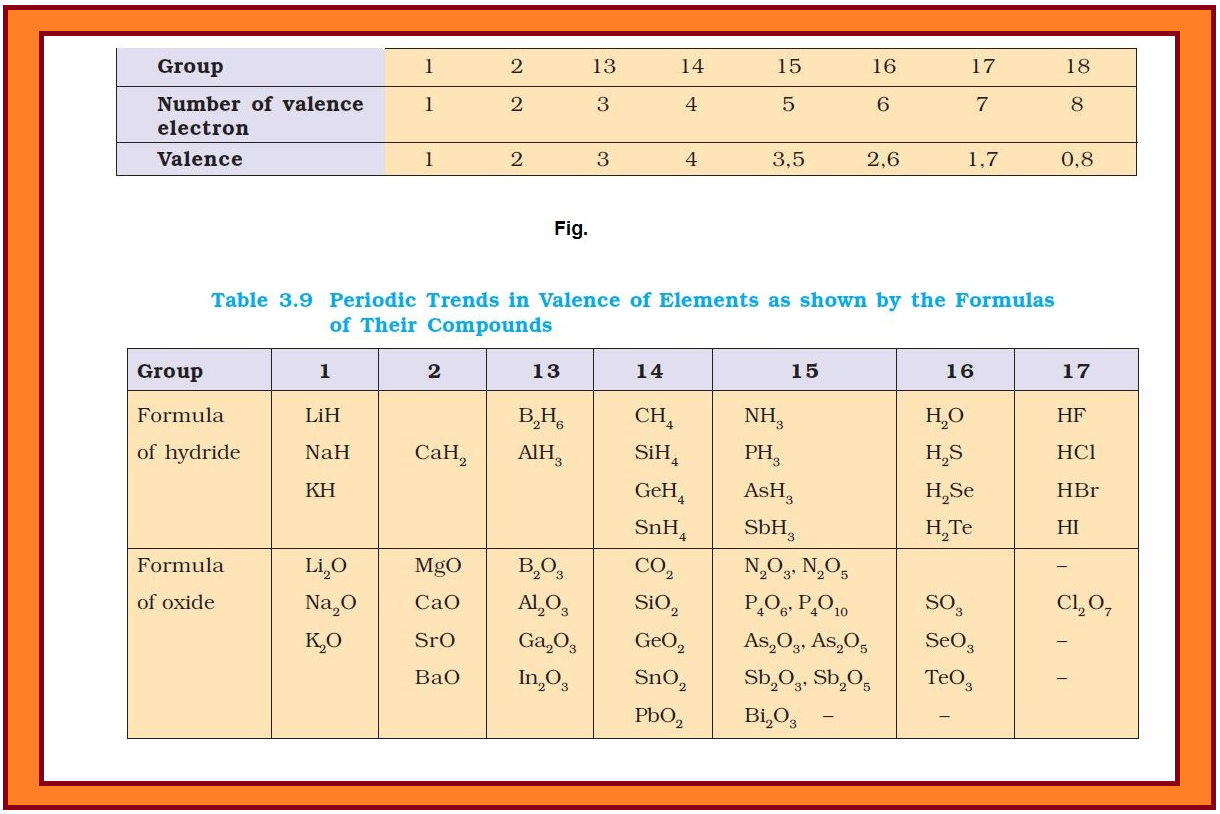
`=>` The valence is the most characteristic property of the elements and can be understood in terms of their electronic configurations.
`=>` The valence of representative elements is usually (though not necessarily) equal to the number of electrons in the outermost orbitals and/or equal to eight minus the number of outermost electrons as shown in fig.
`=>` Nowadays the term oxidation state is frequently used for valence.
`=>` Consider the two oxygen containing compounds : `OF_2` and `Na_2 O`.
● The order of electronegativity of the three elements involved in these compounds is `F > O > Na`.
● Each of the atoms of fluorine, with outer electronic configuration `2s^2 2p^5`, shares one electron with oxygen in the `OF_2` molecule.
● Being highest electronegative element, fluorine is given oxidation state `–1`.
● Since there are two fluorine atoms in this molecule, oxygen with outer electronic configuration `2s^2 2p^4` shares two electrons with fluorine atoms and thereby exhibits oxidation state `+2`.
● In `Na_2O`, oxygen being more electronegative accepts two electrons, one from each of the two sodium atoms and, thus, shows oxidation state `–2`.
● On the other hand sodium with electronic configuration `3s^1` loses one electron to oxygen and is given oxidation state `+1`.
● Thus, the oxidation state of an element in a particular compound can be defined as the charge acquired by its atom on the basis of electronegative consideration from other atoms in the molecule.
`=>` Some periodic trends observed in the valence of elements (hydrides and oxides) are shown in Table 3.9.
`=>` There are many elements which exhibit variable valence.
● This is particularly characteristic of transition elements and actinoids.
`=>` The valence of representative elements is usually (though not necessarily) equal to the number of electrons in the outermost orbitals and/or equal to eight minus the number of outermost electrons as shown in fig.
`=>` Nowadays the term oxidation state is frequently used for valence.
`=>` Consider the two oxygen containing compounds : `OF_2` and `Na_2 O`.
● The order of electronegativity of the three elements involved in these compounds is `F > O > Na`.
● Each of the atoms of fluorine, with outer electronic configuration `2s^2 2p^5`, shares one electron with oxygen in the `OF_2` molecule.
● Being highest electronegative element, fluorine is given oxidation state `–1`.
● Since there are two fluorine atoms in this molecule, oxygen with outer electronic configuration `2s^2 2p^4` shares two electrons with fluorine atoms and thereby exhibits oxidation state `+2`.
● In `Na_2O`, oxygen being more electronegative accepts two electrons, one from each of the two sodium atoms and, thus, shows oxidation state `–2`.
● On the other hand sodium with electronic configuration `3s^1` loses one electron to oxygen and is given oxidation state `+1`.
● Thus, the oxidation state of an element in a particular compound can be defined as the charge acquired by its atom on the basis of electronegative consideration from other atoms in the molecule.
`=>` Some periodic trends observed in the valence of elements (hydrides and oxides) are shown in Table 3.9.
`=>` There are many elements which exhibit variable valence.
● This is particularly characteristic of transition elements and actinoids.